This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Intentional defects make for better reactions, researchers report
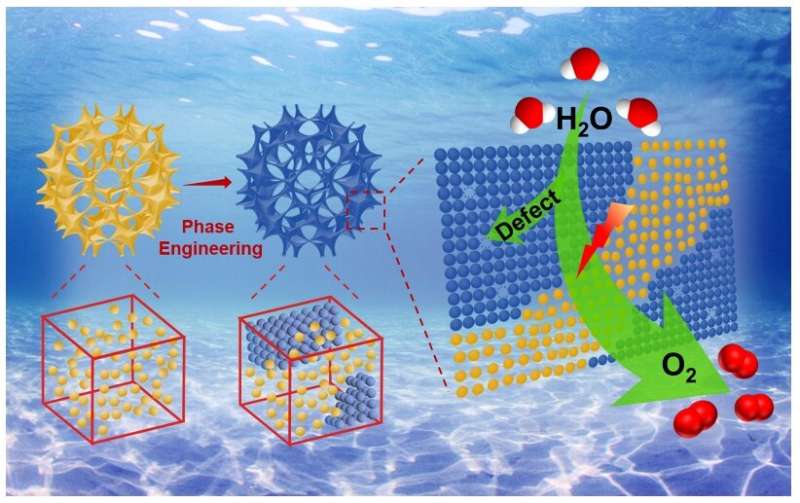
A defect is not always a bad thing. In fact, when it comes to improving the electrocatalysis process that produces clean-burning hydrogen gas, it may be a very good thing. Researchers based in China engineered an electrocatalyst—which speeds up a desired reaction—with both amorphous and crystalline architectures that contains defects in the atomic structure. The defects enable the electrocatalyst to trigger "superior" reaction activity, the team reported.
They published their results Nano Research Energy.
"Hydrogen generation from water electrolysis—or using electric current to split water to separate hydrogen from oxygen—driven by renewable energy is a promising technology in mitigating and solving the crisis of energy and environment," said Cuiling Li, a professor at the Chinese Academy of Sciences' Technical Institute of Physics and Chemistry who is also affiliated with the Beijing Institute of Technology and the Binzhou Institute of Technology.
Oxygen evolution reaction is the anodic reaction of water electrolysis, in which direct current causes a chemical reaction that splits the oxygen molecules from the water molecules. However, Li called this reaction "a sluggish process," and it limits water electrolysis as a sustainable mechanism to produce hydrogen gas. According to Li, the oxygen evolution reaction is slow because it requires a lot of power to trigger how the molecules transfer their constituents, but it could be sped up with less power if integrated with more efficient catalysts.
"Exploiting efficient electrocatalysts for the oxygen evolution reaction is paramount to the development of electrochemical devices for clean energy conversion," Li said.
The researchers turned to ruthenium oxide, a lower cost catalyst that adheres less to reactants and intermediates than other catalysts.
"Ruthenium oxide-based nanomaterials with better oxygen evolution reaction performance in comparison to commercial products have been reported, while more sophisticated electrocatalyst design strategies to evoke more efficient catalytic performance are urgently required and largely unexplored," Li said.
To fill this gap, the researchers synthesized ruthenium oxide porous particles. They then treated the particles to produce rationally regulated heterophases, meaning the particles contain different architectures integrated together. The porous and heterophase structure provides the defects—essentially nicks in the atomic structure—which enable more active sites for the oxygen evolution reaction to proceed with more efficiency, according to Li.
"Benefitting from the abundant defects, crystal boundaries and active site accessibility of the resultant samples, superior oxygen evolution reaction performance was demonstrated," Li said, explaining that the engineered electrocatalysts not only produces a better oxygen evolution reaction, but it also does with less electricity powering the process. "This study demonstrates the importance of phase engineering and provides a new pathway for the design and synthesis of strategies-combined catalysts."
More information: Chengming Wang et al, Phase engineering oriented defect-rich amorphous/crystalline RuO 2 nanoporous particles for boosting oxygen evolution reaction in acid media, Nano Research Energy (2023). DOI: 10.26599/NRE.2023.9120070
Provided by Tsinghua University Press