This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unlocking the secrets of cilia: NSL complex regulates intraciliary transport system
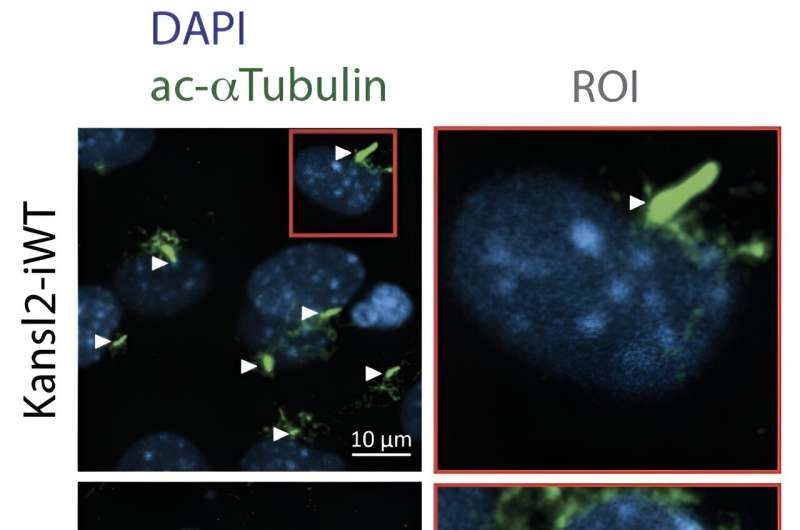
Cilia are thin, eyelash-like extensions on the surface of cells. They perform a wide variety of functions, acting as mechanosensors or chemosensors, and play a crucial role in many signaling pathways.
In the last few decades, the organelle has undergone a remarkable, but at the same time sinister, career transformation. It evolved from an organelle whose relevance was unclear to becoming a central player in the pathogenesis of a large group of diseases.
These so-called ciliopathies are associated with a wide range of symptoms, including hearing loss, visual impairment, obesity, kidney disease, and mental disability. Different gene mutations impair cilia formation, maintenance, and function, resulting in these ciliopathies, which can sometimes be multi-organ, syndromic disorders.
The proper assembly, maintenance, and function of cilia rely on a process called "intraciliary transport." Components of the intraciliary transport system "walk" on the microtubule to deliver cargo between the cell body and the ciliary tip to ensure a constant supply of materials.
Mutation of genes encoding components within the intraciliary transport machinery could lead to ciliopathies. In their recent study in the journal Science Advances, the lab of Asifa Akhtar identified the NSL complex as a transcriptional regulator of genes known for their roles in the intraciliary transport system of cilia across multiple cell types.
The NSL complex enables intraciliary transport
The NSL complex is a potent epigenetic modifier that regulates thousands of genes in fruit flies, mice, and humans. However, most of the functions of the NSL complex remain mysterious and have only recently begun to be elucidated. "Previous research from our lab indicates that the NSL complex controls many pathways critical for organismal development and cellular homeostasis," says Akhtar, Director at the MPI of Immunobiology and Epigenetics in Freiburg.
The complex comprises several proteins and is a histone acetyltransferase (HAT) complex that prepares the genes for activation. "Think of gene regulation as a team effort with different players. One important player is the NSL complex. It puts special marks on the histone proteins on which the DNA is wrapped around in the nucleus, like putting up green flags. These flags tell other regulators to switch on specific genes. We now found that the NSL complex does exactly this for a group of genes linked to moving materials within cilia," says Tsz Hong Tsang, the first author of the study.
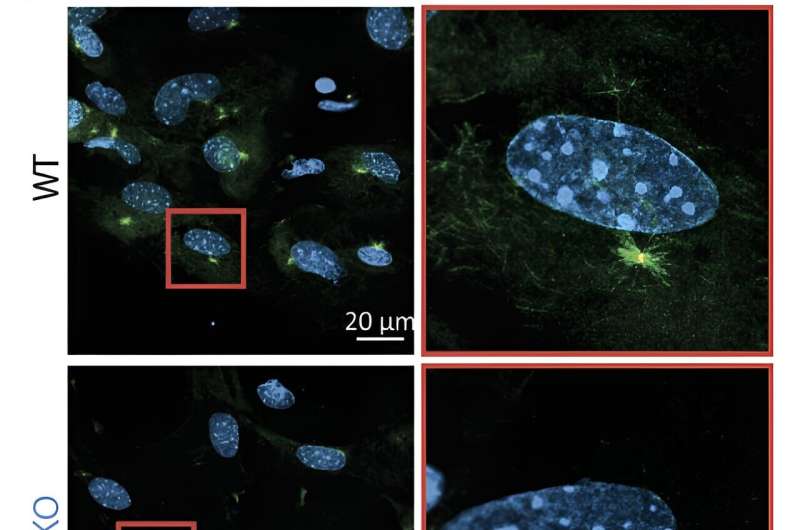
Without components of the NSL complex, the cell cannot build a cilium
The intraciliary transport system is essential because it is needed to build a functional cilium. The cell uses the intraciliary transport system to move material from the cilium base to the growing tip—similar to building a tower. In the study, the researchers used mouse cells to determine the functional consequences of the loss of the NSL complex in the cells.
They found that fibroblast cells lacking the NSL complex protein KANSL2 could not activate the transport genes nor assemble cilia. "As cilia are the sensory and signaling hubs for cells, loss of KANSL2 leads to the inability of cells to activate the sonic hedgehog signaling pathway, which plays important roles in the regulation of embryonic development, cell differentiation, and maintenance of adult tissues as well as cancer," says Akhtar.
Although tiny protrusions, these sensory organelles are incredibly important to cells. Ciliopathies, which affect organs as diverse as the kidney, liver, eye, ear, and central nervous system, remain challenging for biological and clinical studies. The researchers at the Max Planck Institute in Freiburg hope that their analysis of the role of the NSL complex has provided important insights into the regulation of these organelles and the genes associated with them, thus contributing to human health.
Consequences of NSL loss in non-ciliated cells
Cilia are found in most cell types in the human body. This explains why ciliopathies can affect so many different organs and tissues, but there are also cells that are not ciliated. One of the cell types that do not have cilia is mature glomerular podocytes, which are special filtration cells in the kidney. "Interestingly, we found that podocytes also express these intraciliary transport genes that are regulated by the NSL complex. So, we wondered what would happen if they are unable to switch on these genes," says Tsz Hong Tsang.
The researchers found that in non-ciliated mouse podocytes, the loss of KANSL2 leads to changes in microtubule dynamics in the cells. Microtubules are cytoskeletal components responsible for the mechanical stabilization of the cell and intracellular transport between different organelles.
While lacking cilia, mature podocytes have specialized cell processes extending from the cell body called primary and secondary processes, whose functions rely heavily on cytoskeletal components. Although apparently milder than the defect in ciliated cells, the Akhtar lab found that the cytoskeletal defects are likely the cause of severe glomerulopathy and kidney failure observed in mice lacking the NSL complex. These and other extraciliary functions of intraciliary transport genes may help explain the complexity of symptoms presented by ciliopathies.
More information: Tsz Hong Tsang et al, Transcriptional regulation by the NSL complex enables diversification of IFT functions in ciliated versus nonciliated cells, Science Advances (2023). DOI: 10.1126/sciadv.adh5598. www.science.org/doi/10.1126/sciadv.adh5598
Journal information: Science Advances