This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study investigates causes of cation pattern formation, with implications for energy applications
A study led by researchers at the Department of Energy's Oak Ridge National Laboratory could uncover new ways to produce more powerful, longer-lasting batteries and memory devices.
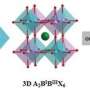
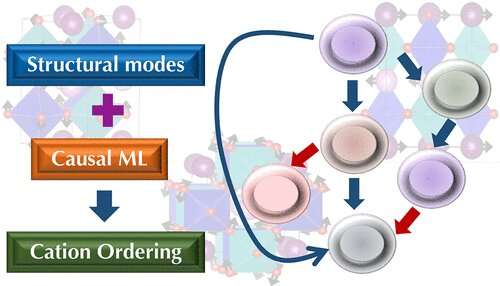
The research examines the causes behind the ordering, or pattern formation, of ions that carry a positive charge, also called cations, in double perovskite oxides, a type of metal considered promising as a potential source of cleaner, more sustainable energy for its magnetism and ability to conduct electricity. The paper, titled "Insights into Cation Ordering of Double Perovskite Oxides from Machine Learning and Causal Relations," is published in Chemistry of Materials.
"If we can understand the fundamental mechanism behind these properties, then we could attempt to grow or otherwise create these perovskite materials for such applications as batteries, memory devices and capacitors," said Ayana Ghosh, an ORNL research scientist and the study's lead author. "We've developed a formula from this study that we'll give to the rest of the world."
Ghosh and researchers from the SRM Institute of Science and Technology in Chennai, India, sought to determine how the patterns formed by cations affect stability in double perovskites. The more stable the material, the better suited it is for potential energy applications.
"This cation ordering can be affected by a number of variables," Ghosh said. "Sizes of the ions matter. Distortions come into play. We wanted to know: Is there one single factor that would drive cation ordering in this kind of system? If so, what is that factor?"
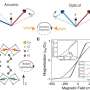
The team relied on resources from two DOE Office of Science user facilities—data collected at ORNL's Center for Nanophase Materials Sciences and time on the Cori supercomputer at Lawrence Berkeley National Laboratory's National Energy Research Scientific Computing Center—to develop a new computational framework. The system combined causal analysis and traditional machine learning with density functional theory, which estimates materials' electronic and atomic structures. Researchers trained the algorithm on various cation types and patterns within double perovskite systems to predict conditions that lead to specific ordering of cations.
The team combined the cause-and-effect relationships observed with the findings from standard predictive machine learning algorithms. Analysis of the results identified trilinear coupling, an interaction among three types of particles, as the necessary condition behind clear layered ordering—one of the essential patterns of cation ordering.
Trilinear coupling combines three types of structural modes that push the cations through the necessary phases to result in properties such as multiferroicity, the combination of magnetization and polarization that makes perovskites promising for energy applications.
"If you have this type of coupling, you should have the formation of clear layered ordering," Ghosh said. "The ordering won't occur without it. You can think of the three modes as fundamental building blocks. This wasn't known before."
Next steps include applying those findings to identify conditions necessary for other types of ordering to design new phases of double perovskite oxides.
"This new insight gives us a road map to go beyond what we've already known from theory," Ghosh said. "Now we can explore further based on these principles."
More information: Ayana Ghosh et al, Insights into Cation Ordering of Double Perovskite Oxides from Machine Learning and Causal Relations, Chemistry of Materials (2022). DOI: 10.1021/acs.chemmater.2c00217
Journal information: Chemistry of Materials
Provided by Oak Ridge National Laboratory