This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Low-pH-dependent RNA binding and oligomerization of SID-1 transmembrane family proteins: Implications for RNA transport
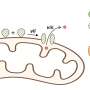
In C. elegans, the protein SID1 plays a crucial role in the systemic RNA interference process by facilitating the transport of exogenous double-stranded RNA into the cytoplasm. Previously, Chen-Yu Zhang's group has already demonstrated that intact plant miRNA found in dietary sources can be absorbed through the mammalian digestive system and mediate cross-kingdom gene regulation.
Mammalian SID-1 transmembrane family proteins, namely SIDT1 and SIDT2, have attracted considerable attention due to their role in facilitating the uptake of regulatory exogenous small RNAs, such as small interfering RNA (siRNA) and plant-derived microRNA (miRNA). Notably, studies using SIDT1-deficient (Sidt1−/−) mouse models have revealed that in gastric pit cells, SIDT1 facilitates the cellular uptake of dietary miRNAs.
Although accumulating evidence shows the involvement of SID-1 transmembrane family proteins in mediating nucleic acid uptake, questions have persisted regarding the precise structural and molecular mechanisms underlying how these proteins enable the uptake of exogenous small RNAs, particularly in low-pH conditions.
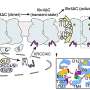
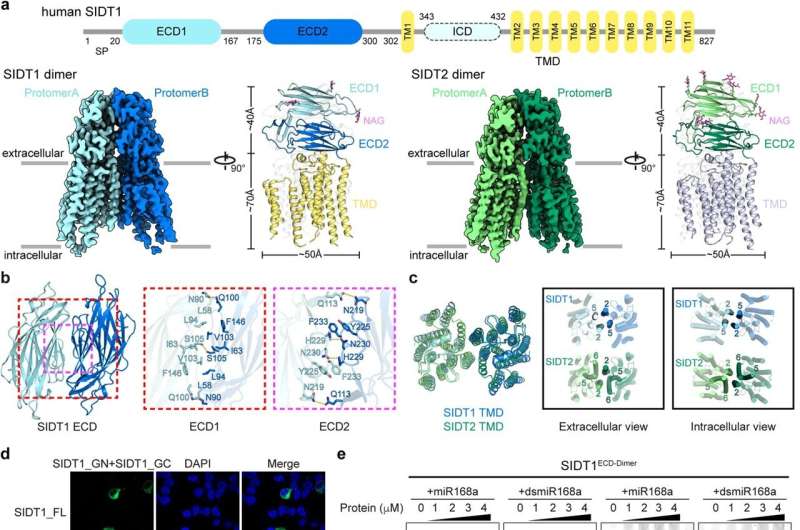
In the present study, the researchers determined the cryo-EM structures of human SIDT1 and SIDT2, unveiling their overall architecture as dimers with remarkable structural congruence. Both the extracellular domains (ECDs) and transmembrane domains (TMDs) contribute to dimer formation.
Notably, the study highlighted the existence of SIDT1 and SIDT2 in dimeric or higher-order oligomeric states, with the TMDs playing a critical role in maintaining these dimeric assemblies in situ. Furthermore, the ECDs of SIDT1 and SIDT2 can efficiently bind to small RNAs in a pH-dependent manner. Under acidic conditions, the ECDs exhibited increased binding affinity to small RNAs, which triggered the higher-order oligomerization of the ECDs.
The significance of this study lies in unraveling the molecular basis of RNA uptake by SIDT1 and SIDT2. Understanding the pH-dependent RNA binding and oligomerization of SIDT1 and SIDT2 provides insights into their functional regulation, particularly in the acidic microenvironments where they are predominantly localized.
This work is important for the following reasons:
- This study presents a comprehensive characterization of the architectural features of human SIDT1 and SIDT2, which exist as dimers in vitro and form dimers or higher-order oligomers in their natural environment.
- ECDs of SIDT1 and SIDT2 can efficiently bind to small RNAs under acidic conditions, indicating a pH-dependent mechanism for RNA binding and uptake. This finding overturns previous claims that SIDT1ECD and SIDT2ECD can only bind longer double-stranded RNAs (>100 bp).
- This study highlights the role of RNA in triggering ECD oligomerization under acidic conditions, shedding light on the potential importance of oligomerization in their RNA uptake process.
- This study introduces a model where nucleic acid-induced oligomerization may form an RNA transport supercomplex involving other partners under low pH conditions.
"SID-1 transmembrane family proteins were first identified as nucleic acid transporters. The ECD regions, however, appear to function as novel nucleic acid binding proteins, raising the hypothesis that these proteins function as membrane-bound RNA binding proteins," Chen-Yu Zhang said.
"Our study highlights the role of RNA in triggering ECD oligomerization under acidic conditions. Moreover, we propose that the phospholipase activity in the TMD might influence the fluidity of the cell membrane system, shedding light on the potential importance of oligomerization in the process of RNA uptake," Xiaoyun Ji added.
The paper is published in the journal Cell Research.
More information: Le Zheng et al, Cryo-EM structures of human SID-1 transmembrane family proteins and implications for their low-pH-dependent RNA transport activity, Cell Research (2023). DOI: 10.1038/s41422-023-00893-1
Journal information: Cell Research
Provided by Nanjing University School of Life Sciences